Description:
More art for my latest fanfic, “Beyond the Eastern Sky”
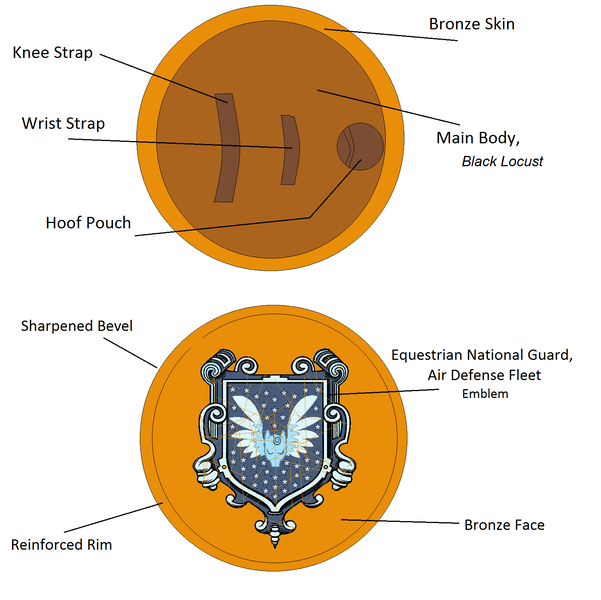
Based on a modified hoplon shield of ancient Greek warriors (the hoplites), Silver Strike’s shield is made of the extremely tough and rot-resistant black locust wood and hard bronze sheet, and is about a half meter in diameter. He can’t cover his entire body behind it, but the shield is extremely durable and is excellent at deflecting heavy blows. The two legs straps and hoof pouch give the wearer precision control over its use.
The emblem on the face is descended from the old Celestine Junta, the military stratocracy of the Pegasi before Equestria’s unification, and is now the symbol of the Pegasus Air Defense Fleet.
Diameter: 50 cm
Thickness: 2 cm wood body, 1 mm bronze sheet
Total Weight: 2 kg
Main Body: Black Locust wood, cross-laminated & riveted together
Face & Rim: High-tin phosphor bronze
80.0% Cu, 16.0% Sn, 2.50% Fe, 1.0% Cr, 0.5% P
The metal alloy employed here represents Equestria’s most advanced bronze alloy in this era. While copper and tin are dirt cheap in Equestria, this alloy is very expensive due to the precision control needed in its production. It is generally limited to military hardware, but it can meet or beat virtually all existing steels of this era for strength, elasticity, hardness, and durability. (And beat a few modern steels, too; this alloy should have mechanical properties rivaling AISI 1060 to 1080 carbon steel, even after heat treatment.)
The discovery of phosphorus as an element revolutionized the manufacture of bronze alloys. Phosphorus removes any remaining copper oxides and sulfides from the alloy, floating these compounds out of the molten metal with other fluxes. In untreated bronze, these oxides and sulfides cause brittleness and greatly weaken the final alloy. The fully deoxidized metal is called phosphor bronze and generally has double the yield strength of untreated tin bronze.
When cast and slow-cooled, copper can only only dissolve up to 8% tin by weight, limiting its potential. Adding more tin than this creates a copper-tin intermetallic compound, Cu31Sn8, which segregates into a network of hard but brittle crystals all throughout the metal. This is because of the extreme difference in melting points for the two metals: 1085*C for copper, 232*C for tin. This segregation can be partially overcome by heat treatment. If the metal is re-heated after casting to 700-800 degrees Celsius for several hours, then quenched in cold water, copper can dissolve up to about 12% of its weight in tin without creating intermetallic crystals. In the best case scenario, the alloy can hold up to 16% tin for maximum strength and hardness without sacrificing its elasticity and durability, but this requires some fancy tricks. See Solid Solution Strengthening
Chromium melts at about 1905*C, iron at about 1540*C, and neither element has appreciable solubility in solid copper or tin. If small amounts are dissolved into molten copper or bronze and then quickly frozen, the chromium and iron will precipitate first, forming innumerable, finely dispersed seed grains of “ferrochrome” crystals as well as phosphide compounds of both elements (assuming phosphorus is present). These seed crystals, combined with very rapid freezing, refines the grain structure of the bronze alloy and inhibits the formation of brittle intermetallics, up to the maximum of 16% tin. See Grain Boundary Strengthening
Additionally, the finely dispersed particles of ferrochrome, iron phosphide, and chromium phosphide all increase the bronze alloy’s tensile strength by inhibiting plastic deformation. When the metal is strained, whether by bending, stretching, or impact, these particles help the metal return to it’s original shape, making it springier. In addition, because these particles are very hard, the wear resistance of the alloy is increased. In great quantities, however, these particles can themselves reduce the metal’s toughness and cause brittleness, so the right balance must be struck. See Precipitation Hardening
Trace amounts of other elements ( <0.25% ) include nickel, manganese, magnesium, zinc, lead, arsenic, antimony, bismuth, etc. These contaminants were never removed from the final bronze alloy, but are present in such small quantities that they typically don’t harm the mechanical properties of the finished metal.
Finally, the mechanical properties of the bronze alloy are ultimately decided by how much cold working goes into the final product. Cold work involves hammering, rolling, drawing, or otherwise reducing the metal’s cross-sectional area while it’s cold or at room temperature, not hot. This is also called strain hardening, and beating the metal into its final shape makes it strong and hard, but at the cost of toughness and durability. Whether the final product is a sword, ring maille, or armor plate, the right balance must be struck for optimum results.
One feature unique to many copper alloys is their resistance to extreme cold. In fact, copper alloys containing up to 38% zinc, 16% tin, 9% aluminum, or 5% silicon actually get stronger and tougher with decreasing temperature. When cold-worked at cryogenic temperatures, like that of liquid nitrogen, these brasses and bronzes can achieve up to four times the yield strength and two times the ultimate tensile strength as they would if worked at room temperature This is due to extreme twinning that occurs in these alloys in such severe cold. Twinned grains help the metal resist deformation and are make it likely to fail gracefully rather than fracture or shatter. See Stacking-fault Energy
(While this phosphor bronze alloy does not exist in real life — or at least, does not have any modern-day industrial standard representing it — I didn’t pull it completely out of my butt. It is based on the copper-chromium alloy C18200 , the copper-iron-phosphorus alloy C19400 and the phosphor bronzes C52400 & C52480. I increased the percentage of tin from 10% to 16% based on tin’s temperature-dependent solubility in copper, and then extrapolated it’s tensile strength and ductility after cold work from there. Hopefully, my extrapolations aren’t too far off the mark. =D)
1975 patent describing the grain-refining effect of chromium and iron in phosphor bronze.
2007 paper describing the strengthening effect of low stacking-fault energy in severely deformed brasses.
2012 paper describing the strengthening effect of low stacking-fault energy in severely deformed aluminum bronzes.
2011 paper describing the strengthening effect of cryogenic cold-rolling in aluminum bronzes.
2008 paper describing a high-tin bronze, produced by spray-forming, free of segregation and copper-tin intermetallics.
2008 paper describing the differences in high-tin bronze produced by spray-forming, and those by conventional casting.
But how can a Bronze Age/ early Iron Age society control metal content and purity so precisely?
Oh, that’s easy.
Magic. :XD:
Special Thanks to :iconlord-giampietro: for letting me use his Pegasopolis Coat-of-Arms
